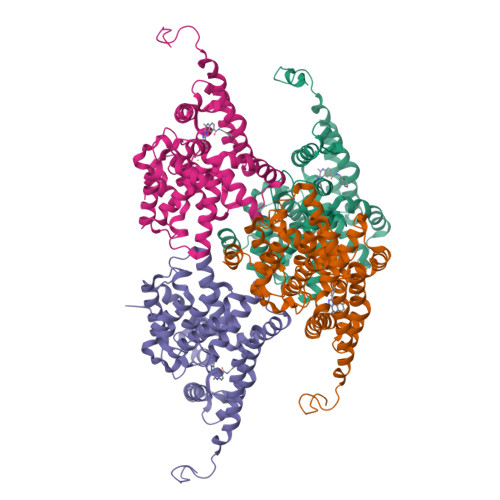
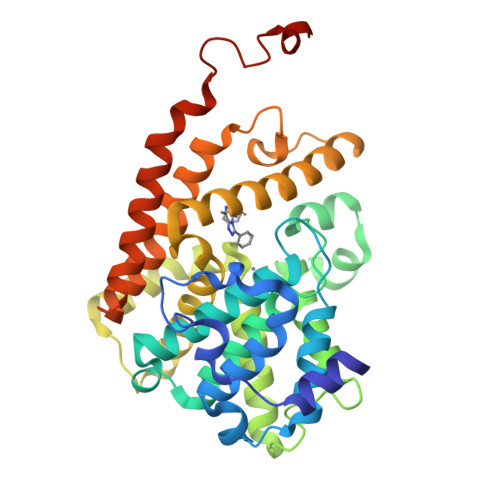
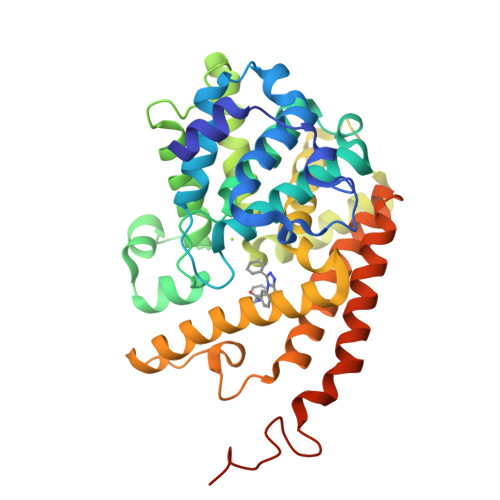
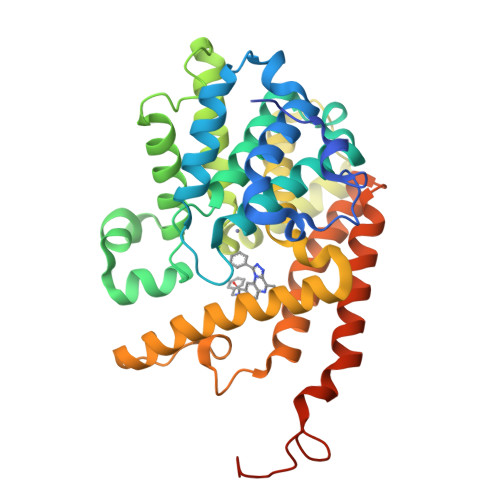
Structure-Based Design of a Potent, Selective, and Brain Penetrating Pde2 Inhibitor with Demonstrated Target Engagement.
Buijnsters, P., De Angelis, M., Langlois, X., Rombouts, F.J.R., Sanderson, W., Tresadern, G., Ritchie, A., Trabanco, A.A., Vanhoof, G., Roosbroeck, Y.V., Andres, J.(2014) ACS Med Chem Lett 5: 1049
- PubMed: 25221665
- DOI: https://doi.org/10.1021/ml500262u
- Primary Citation of Related Structures:
4D08, 4D09 - PubMed Abstract:
Structure-guided design led to the identification of the novel, potent, and selective phosphodiesterase 2 (PDE2) inhibitor 12. Compound 12 demonstrated a >210-fold selectivity versus PDE10 and PDE11 and was inactive against all other PDE family members up to 10 μM. In vivo evaluation of 12 provided evidence that it is able to engage the target and to increase cGMP levels in relevant brain regions. Hence, 12 is a valuable tool compound for the better understanding of the role of PDE2 in cognitive impairment and other central nervous system related disorders.
Organizational Affiliation:
Neuroscience Medicinal Chemistry, Janssen Research & Development, a Division of Janssen Pharmaceutica NV , Turnhoutseweg 30, 2340 Beerse, Belgium.